What Would it Take for Bird Flu to Go Pandemic?
How H5N1 could make the jump and what we can do to stop that

Whenever something comes up in bird flu news I think about what this means for the ability of H5N1 to cause a pandemic, because immediately that’s what I’m going to be asked. Everyone wants to know if H5N1 is going to cause a pandemic. Nobody, including me, knows. We only know that it could.
To cause a pandemic, H5N1 needs to gain the ability to transmit efficiently from human-to-human by the respiratory route. It only needs to retain some of its pathogenicity in people to kill millions more than COVID did. I’ve spent a lot of time thinking about the underlying virology: how does the virus need to change to become pandemic-capable and what circumstances could enable those changes?
I gave a talk a couple months back about how the reorganization of the US government and its new pro-virus approach to public health will leave us hopelessly unprepared and vulnerable if H5N1 gains the ability to spread between humans. In August, H5N1 outbreaks hadn’t started ticking back up. Now they are exploding.
Wild birds began migrating in September and they brought their bird flu with them. The US Department of Agriculture Animal and Plant Health Inspection Service (USDA/APHIS) reports active confirmed outbreaks in 42 flocks affecting 6.3 million birds across 12 states. Some of these outbreaks are occurring in huge chicken or turkey operations with thousands or even millions of birds. In Canada, there are 13 active outbreaks, including the accursed ostriches in BC, across 5 provinces. There is a lot of H5N1 around in birds that have a lot of contact with people, which means a lot of opportunity for transmission to humans and further adaptation. This is a high risk scenario that will only become more dangerous as seasonal flu begins circulating in people, but it’s very difficult to quantify that risk because there’s so much we don’t know.
I read an article the other day that made me want to scream. It said that we are only one mutation away from a H5N1 pandemic, based on a paper the author read and a prediction they made on social media last year.
This is an extremely simplistic understanding of influenza cross-species adaptation. We don’t know for sure, but there are likely multiple ways the virus will need to change to be transmitted between people and make some of them sick. This is really complex and we are hindered by major knowledge gaps that will only grow as the administration further eviscerates virology research.
Although we don’t actually know all of the changes that would need to occur, we do know in general what viruses need to do certain things in order to make new viruses and establish onward transmission. We also know some things about how viruses make people sick. Viruses need to encounter a human host under the right circumstances and they need to be able to infect and replicate in their host cells, counter host defenses, and shed enough virus from the right tissues that they can transmit to another host. We can look at H5N1 in this context, identify pandemic traits the virus needs to acquire, assess the barriers the virus needs to overcome to acquire these traits, and use this knowledge to inform risk reduction.
Exposure
In order to get infected by a virus, you have to encounter the virus in the physical world under circumstances that facilitate transmission. If you are in a containment lab with a vial of frozen H5N1, it presents a negligible exposure risk even if your PPE fails. You could take off your PAPR and start taking big lungfuls of BSL3 air (don’t do that, actually) and still you wouldn’t get infected because a virus ice cube can’t jump out of a sealed Sarstedt tube into your nostril. Live virus is always handled in a biosafety cabinet, which is like a containment lab within a containment lab. A “lab leak” of H5N1 is unlikely when biosafety best practices are maintained. If you are hooking up the claws of a milking machine to an infected cow’s udders or feeding your commercial flock or collecting eggs from your backyard chickens, your exposure risk is much higher.

In the real world, there are multiple viable exposure routes for H5N1, which is unfortunately everywhere in many host species that live in close proximity to people. The virus can be inhaled, acquired through direct contact with an infected host, indirect contact with fomites (objects or surfaces with infectious virus on them), or ingested in food, water, or milk. The likelihood of exposure depends on the environment, behavior, and circumstances of the population in question, as well as the amount of infectious virus.
Right now, exposure risk is limited to people who have contact with birds, cows, or other infected animals. For H5N1 to become a pandemic virus, humans would need to become an exposure risk themselves. The virus must acquire the ability to spread from person to person by the respiratory route, which means it must adapt to grow and shed in the cells of the upper respiratory tract. The more humans the virus infects, the more opportunities it gets to adapt. We need to stop humans from getting infected now by reducing their exposure risk. This can be done by using PPE such as respirators in high-risk environments like dairy or poultry farms, shoring up biosecurity practices to prevent virus from coming and going off an infected farm, altering work practices when possible to reduce splashing and creating aerosols, training workers, incentivizing reporting, and quarantine and isolation for people who do get exposed or infected.
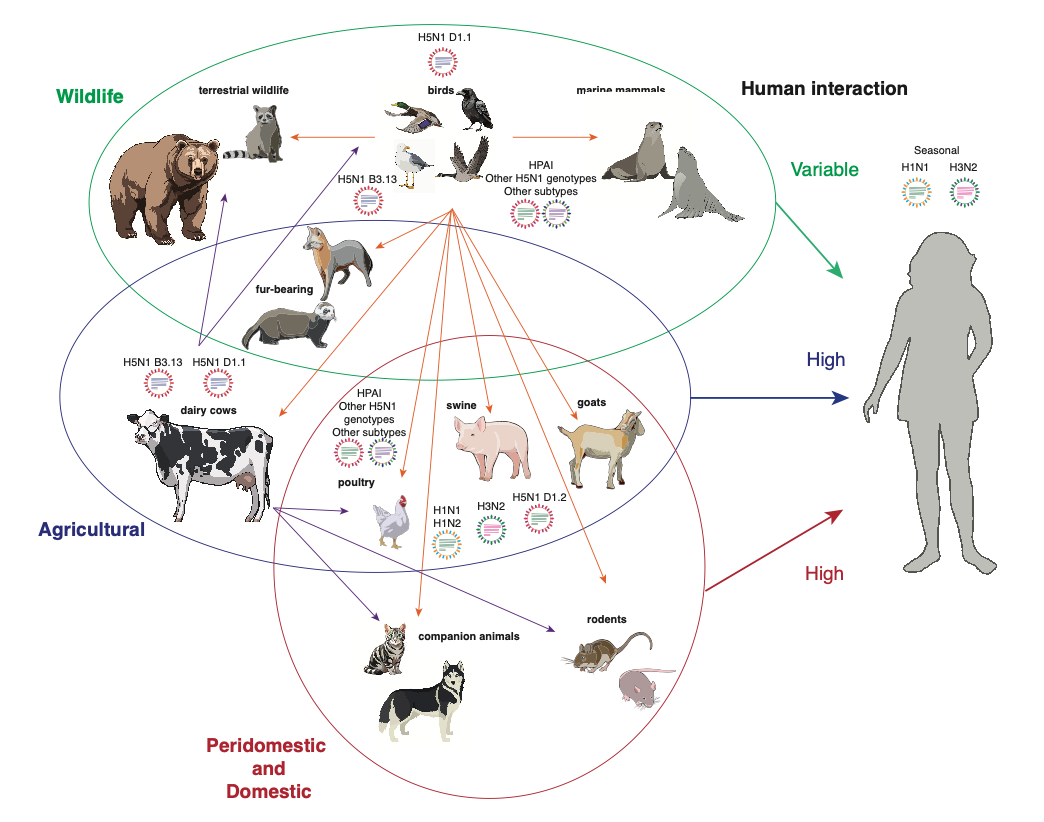
Another way to reduce human exposure is to reduce the number of infected animals that humans come into contact with. There’s not much we can do about wild birds or animals, but we can certainly take more steps to control the cow outbreak (more testing and surveillance, better quarantine procedures, better biosecurity, maybe vaccination), reduce poultry outbreaks (better biosecurity, vaccination), and prevent companion animals like cats from becoming infected (better monitoring of pet food, keeping cats indoors).
Ideally, vaccination could be deployed for humans too, as flu vaccines reduce transmission risk even though they don’t eliminate it. There are 10 million doses of H5N1 vaccine in the national stockpile, which could be used in a targeted vaccination campaign for people with high exposure risks. However, given the current administration’s hostility toward vaccines and the fact that they have effectively stopped testing people who have been exposed, I am not optimistic that this countermeasure will be used.
Pandemic trait to acquire: virus can use human receptor
Virus barrier to overcome: adaptation to growth and shedding from human upper airway
Risk mitigation: reduce exposure risk at the human-animal interface, reduce overall prevalence in animal populations, vaccination of individuals with high exposure risk
Susceptibility
The ability of a virus to infect a cell depends on its ability to get inside the cell, which is determined by the ability of the viral envelope glycoproteins to interact with its entry receptor. Virologists call the ability to infect a cell susceptibility. Enveloped viruses like influenza have a membrane around the virus particle with viral proteins embedded in it. The spike protein of SARS-CoV-2 is an example of an envelope glycoprotein. Influenza viruses have 2 glycoproteins, hemagglutinin (HA) and neuraminidase (NA). These are the H and N in H5N1. HA primarily determines entry and it binds a sugar molecule called sialic acid that is bound to another sugar called galactose that “decorates” host proteins. HA binds sialic acid and unlocks the cell for the virus to enter it.
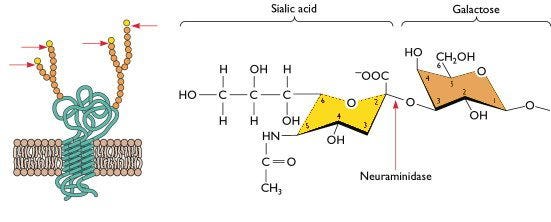
Sialic acid comes in two basic flavors: bird (alpha-2,3-linked) and human (alpha-2,6-linked). H5 HA preferentially binds the bird receptor, since birds express this throughout their respiratory and gastrointestinal tracts. Human seasonal flu viruses bind the human version.
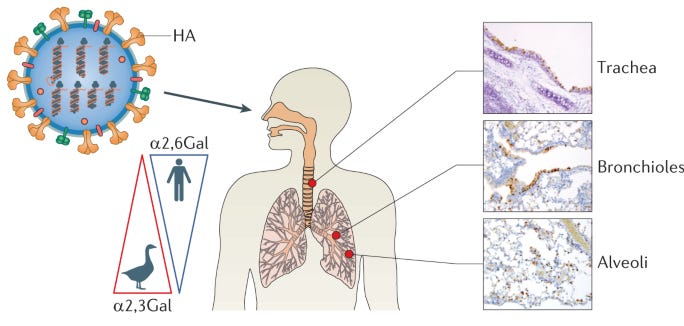
Humans have the bird receptor and vice-versa, they are just distributed differently in different organs and tissues. In humans, the bird receptor is located deep in the lungs and on the cornea of the eyes, while the human receptor is located throughout the respiratory tract. Importantly, it is in the upper respiratory tract, allowing for transmission by aerosols (breathing). This is why humans can spread human-adapted seasonal flu to each other but not bird flu, although they can still get bird flu if exposed to enough virus. When humans breathe in a large enough dose of H5N1, it can establish a lower respiratory tract infection that is basically instant viral pneumonia, but these people will not exhale enough virus to cause an onward infection because they aren’t shedding from the upper respiratory tract and people don’t have the bird receptor there. The big concern is that the virus will acquire the ability to use the human receptor.
A mutation allowing a receptor switch is likely to arise in an infected human. Contrary to the article I disliked, it’s not only HA Q226L (which means a mutation occurred that changes glutamine to leucine in the HA protein at amino acid 226) that can do this. While HA Q226L has been shown in vitro to permit binding of the human receptor and is a clear concern, it’s not the only mutation that can allow this to happen. We don’t know if this mutation alone is enough to enable efficient transmission in a human, but we do know that these mutations can and do accumulate under the right selection pressure (ie: in a human).
In both the Louisiana patient who died of H5N1 and the teenager in BC who almost did, sequence analysis showed emerging mutations in HA associated with binding the human receptor. A virus with a slightly improved ability to use the human receptor can be passed to another human, where it will continue to adapt. A few rounds of human infection could easily select a virus that infects human cells very efficiently as it acquires multiple mutations allowing progressively better binding to the human receptor. Therefore, it is really important to identify and isolate infected humans and deny the virus the opportunity to adapt. However, since human testing and monitoring has fallen off a cliff in the US, we are currently at high risk of missing an early cluster of human cases that might occur.
Other mammals have different distributions of these two receptors and that determines which cells and tissues are susceptible to bird versus human flu. We don’t know that much about it for many species. We do know that pigs have both the bird and the human receptors throughout their respiratory tract, so they are of special concern due to their potential for allowing an avian virus to adapt to using the human receptor. When two flu viruses infect the same host, they can swap genes in a process called reassortment. This can allow a virus to make big evolutionary leaps in a short period of time. Pigs can be infected with both human seasonal flu viruses and avian viruses at the same time, so they present additional risk for reassortment. A receptor switch in pigs accompanied by reassortment can create a pandemic virus. The 2009 H1N1 pandemic was a triple reassortant virus that emerged from pigs.
Pigs generally don’t get very sick from influenza viruses, which creates another problem: how does a farmer identify an infected pig? The answer to that is molecular (with PCR tests) or genomic (with sequencing) surveillance. In addition to identifying human cases, pig surveillance is essential to preventing the emergence of pandemic H5N1 viruses. This is primarily conducted by the USDA/APHIS as part of the Influenza A Virus in Swine (IAV-S) program. The National Animal Health Laboratory Network (NAHLN) provides testing services to swine surveillance efforts conducted by state and federal animal health officials and academics. Surveillance is also bolstered by industry-led initiatives such as the Swine Health Information Center. Unfortunately, surveillance efforts are currently dependent on voluntary submissions from sick pigs rather than proactive testing. Even more unfortunately, cuts to USDA staff have dramatically reduced surveillance capacity. More than 1300 employees have left APHIS, which severely impairs its ability to carry out surveillance at national scale.
Pandemic trait to acquire: virus is capable of infecting human cells in the upper respiratory tract
Virus barrier to overcome: virus can use the human receptor for cell entry
Risk mitigation: increase surveillance in humans and pigs, isolate infected individuals to prevent further adaptation
Permissivity and Fitness
It’s not enough for a virus to enter a cell. It also needs to infect it. Specifically, it needs to replicate its genome and make viral proteins so it can assemble baby viruses (or progeny virions, in virologist terms) that can go on to infect a new host cell. This means it has to interact with the host. When a virus can carry out its replication cycle in a given host cell, we say that cell is permissive to infection. Viruses can’t replicate without “hijacking” their hosts, and permissivity is determined by their hijacking ability.
An extension of permissivity is fitness, which refers to how well the virus replicates in a permissive host. A virus that can replicate a little bit is less likely to cause a pandemic than a virus that can replicate a lot.
In order to replicate, H5N1 has to do two things: make viral RNA and make viral proteins. H5N1 specifically has to make the protein subunits of the viral polymerase (enzyme that copies RNA) that can assemble into a complex to copy the viral gene segments. Complex formation requires a host protein called ANP32. If ANP32 can’t bind the viral polymerase, replication won’t occur.
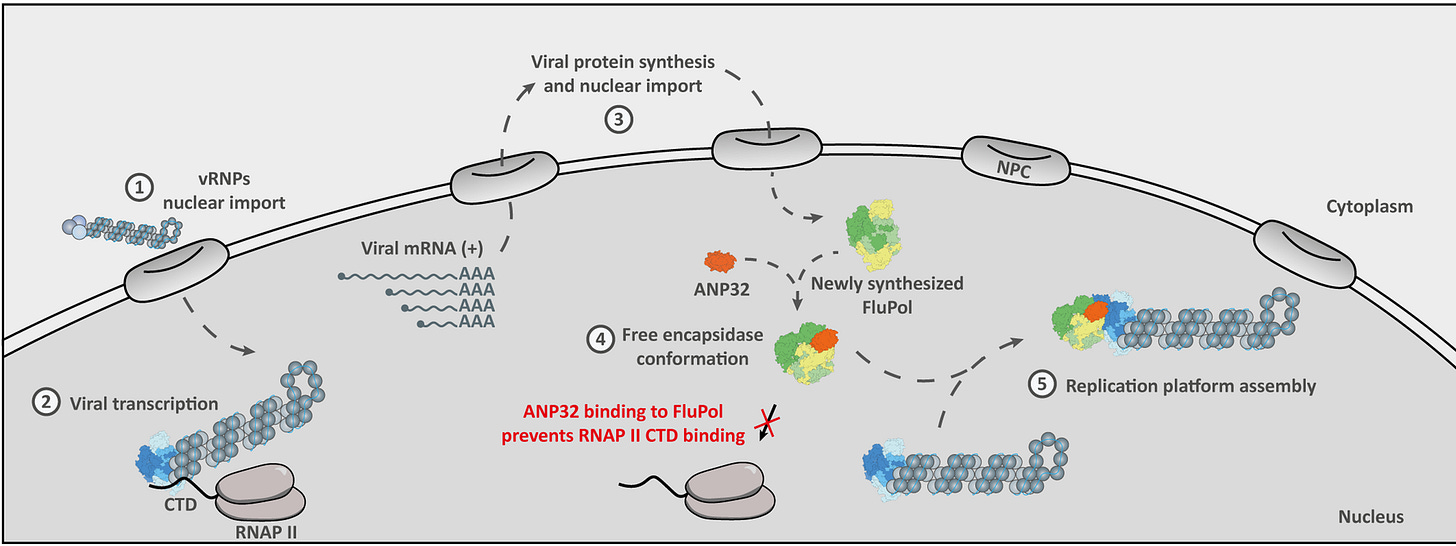
There are several mutations in the PB2 polymerase subunit that are known to enhance fitness in mammalian hosts by increasing their ability to interact with ANP32. Some of these, like PB2 E627K and PB2 D701N, have also emerged in humans. The very first dairy worker identified was infected with a virus with PB2 E627K. We know that these mutations emerge very quickly in human infections and confer a fitness advantage, which can be additive if a virus passes through multiple human hosts. But they alone have not yet allowed the virus to “go pandemic.”
There are likely many more interactions required for replication that influence viral fitness that we don’t know about. Most RNA viruses replicate in the cytoplasm of the cells they are infecting, but flu is weird because it replicates in the nucleus. That means it has to interact with cellular transport and nuclear import machinery to even get in there. Once it’s in the nucleus, there is a whole other process that is delightfully called “cap-snatching” that flu uses to make viral mRNA. The virus also has to selectively shut down host protein synthesis entirely to make all this happen. Most of the virus-host interactions governing these processes are poorly understood, if we even know about them at all. We don’t know all of the adaptive changes the virus will need to make to overcome incompatibilities in these unknown interactions. This is one reason why it is so difficult to quantify the probability of a bird flu pandemic.
One thing that would help would be to know more about these complex interaction networks. Unfortunately, the Executive Order on Improving Biosafety and Biosecurity in Biological Research has severely hindered flu research in the US. Many of the top American flu scientists are not able to do the critical research that might help clarify this because it is no longer allowed. Those of us in other countries are trying, but it’s a lot of work and there is less funding available.
Pandemic trait to acquire: virus can replicate in the human upper respiratory tract
Virus barrier to overcome: virus can interact with host proteins required for replication
Risk mitigation: study virus-host interactions to identify human-adaptive mutations, increase surveillance in humans, isolate infected individuals to prevent further adaptation
Immune Evasion
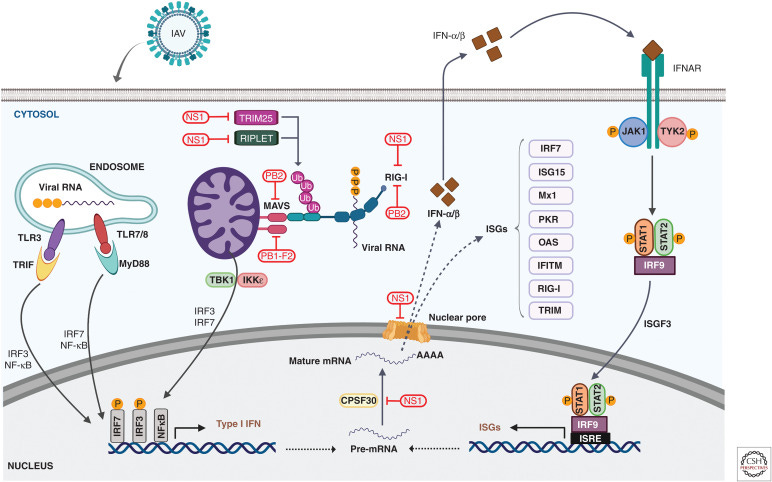
Even viruses that can infect a human host effectively don’t always cause disease. The central hypothesis of my lab’s research is that the host determines this as much as the virus does based on the way it responds to infection. If the host responds by mounting a robust, tightly controlled antiviral response, the virus will be less pathogenic. If the host response is not well-regulated, that means trouble for the host. For highly pathogenic viruses like H5N1, screwing up the host response leads to uncontrolled inflammation and severe disease. Influenza A viruses have a lot of different means of disrupting the interferon pathway (the main innate antiviral response pathway). This is referred to as innate immune evasion or interferon antagonism. It determines pathogenicity.
Viral infections trigger “sensors” in cells that turn on the interferon pathway. This results in interferon being made, which is a cytokine (signaling molecule) that effectively announces to all nearby cells that there’s a virus around and start expressing interferon-stimulated genes (ISGs). ISGs do various things, including regulating inflammation, directing different immune cells where to go and what to do, turning on adaptive immune responses, and directly targeting the virus or steps in its replication cycle. ISGs have a profound impact on proinflammatory cytokines, which are messengers that amp up inflammation. Proinflammatory cytokines are crucial for clearing virus infections, but if they are not kept in check, they lead to widespread disease pathology.
H5N1 has a number of different ways to disrupt these protective host responses. The viral proteins NS1, PB2, and PB1-F2 all block interferon responses at multiple steps in the pathway. NS1 and PB2 interfere with sensors of viral infection like RIG-I, while PB2 and PB1-F2 block its ability to signal infection to the cell. NS1 blocks various restriction factors and prevents interferon, ISGs, and cytokines from being expressed. These viral proteins do this by interacting with the different host proteins that carry out these functions. Sometimes that means that they need to adapt in order to interact with a specific protein. We don’t currently know the degree of adaptation that has occurred, but given how many mammals have died, my guess is that it’s pretty likely these viruses are fairly well adapted in this regard to mammalian hosts.
Not much has been done so far to look at the capacity of clade 2.3.4.4.b H5N1 to evade the innate antiviral response, although my colleagues at NIAID did a nice study comparing two clade 2.3.4.4.b cow viruses (one isolated from a human dairy worker in Texas, one from an infected cow in Ohio) to a historical, highly lethal bird-derived H5N1 from an outbreak in 2004 (H5N1/Vietnam/12/03/2004 or Vn1203 for short). They looked at ISG expression in two different types of human lung organoids (iPSC-derived vs adult stem cell-derived). Organoids are basically lumps of differentiated epithelial cells that approximate some features of an actual lung and are more lung-like than just some undifferentiated epithelial cells on a plate.
The authors looked at how well these three viruses grew and discovered that both cow-derived viruses replicated significantly less than Vn1203, showing that they have a fitness defect in these human lung organoids. They were also less cytopathic (they killed fewer cells). When you look at interferon expression and a proinflammatory cytokine called TNFalpha, you see that there are different patterns of expression, depending on the type of lung organoid infected and the virus.

This doesn’t provide a clear answer about how well the currently circulating H5N1 cow viruses antagonize interferon, since interferon expression is dependent on the source of the lung organoid cells and there were almost no interferon responses in the iPSC-derived lung organoids. The cow-derived viruses both were less fit and less cytopathic than Vn1203, so that further confounds this comparison. However, the lack of interferon induction in the iPSC-derived lung organoids and the Texas cow-derived human isolate indicate that the infections are evading sensors that trigger antiviral responses. It also suggests that in both types of lung organoids, inflammation gets cranked up. That indicates that all of these viruses have some capacity to be highly pathogenic.
We have only scratched the surface of understanding the currently circulating H5N1 viruses’ capacity for immune evasion. We need to do a lot more research to understand how these responses are regulated (I’m working on it). Going forward, we need to both do experiments to understand the mechanisms by which these viruses might be evading immunity and how this leads to pathogenesis. If we understand more about this, we can identify ways to modulate the host response (treating with different drugs targeting the host, for example) that might impair H5N1’s ability to antagonize the antiviral response or trigger uncontrolled inflammation.
Pandemic trait to acquire: virus evades or antagonizes host antiviral responses
Virus barrier to overcome: virus can interact with host proteins involved in triggering protective responses and disrupt their function
Risk mitigation: study virus-host interactions to identify mechanisms of shutting down interferon, identify immunomodulatory drugs that could ameliorate disease severity by altering host responses
Assembly and Egress
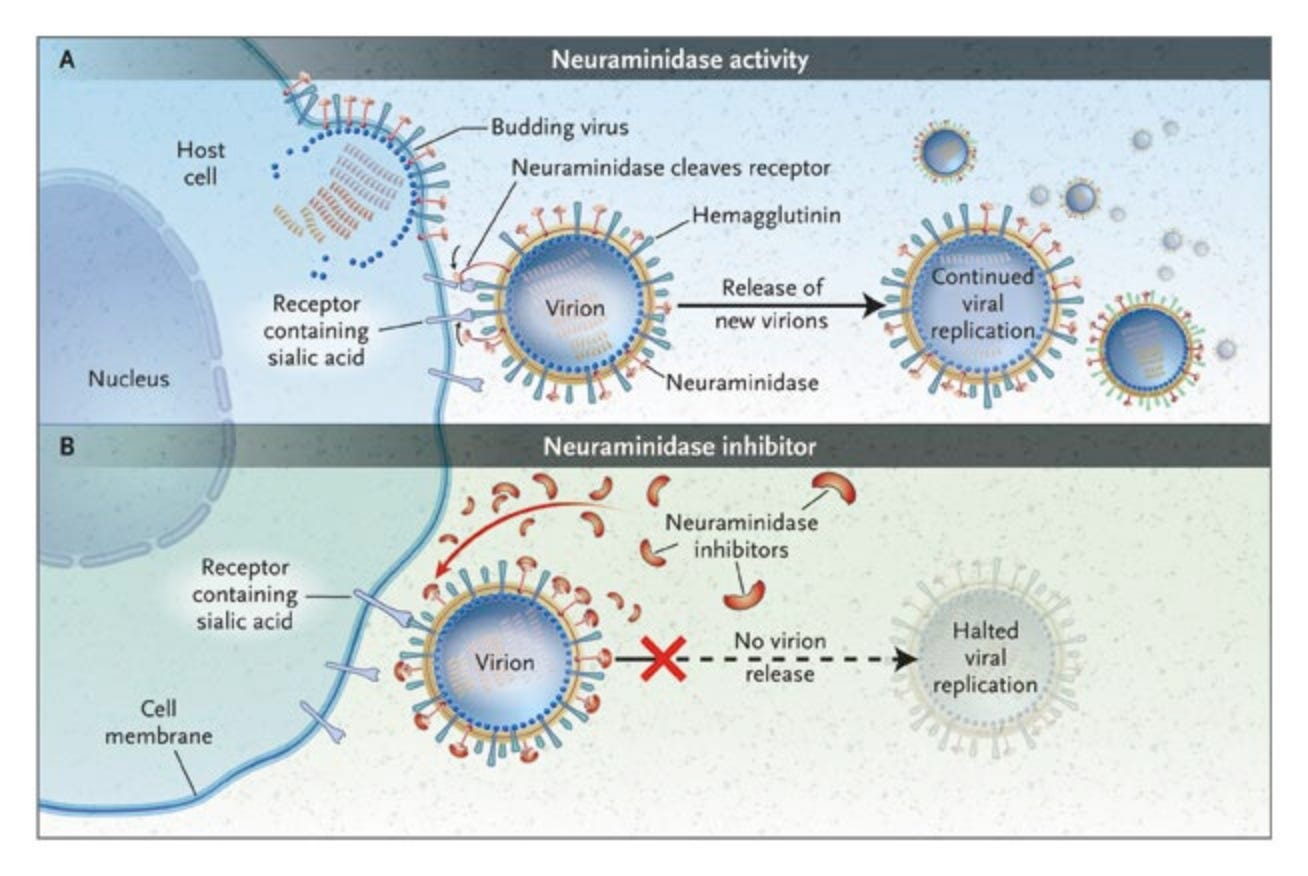
In addition to getting into cells, copying their genomes, making viral proteins, and subverting the host response, viruses also need to physically make new virus particles (assembly), which then need to leave the cell (egress) so they can go on to infect another. To make virus particles, all 8 of the viral gene segment and the structural proteins need to be transported to the cell membrane. The particles assemble through a series of complex interactions we don’t fully understand, then bud from the membrane. The HA on these new budding viruses will bind receptors on the cell surface, so the NA protein in the viral envelope has to sever these connections to release the virion from the cell surface. Then it can go on to infect another cell.
One of the only two classes of drugs for treating flu, NA inhibitors (like oseltamivir/TamiFlu) blocks this interaction. If the virus particles can’t leave their host cell, the infection can’t spread. Unfortunately, resistance mutations can arise in the virus pretty easily that render NA inhibitors fairly useless.
We don’t know much about how well-adapted clade 2.3.4.4.b H5N1 is to carrying out assembly and egress efficiently in human cells, particularly in the upper respiratory tract. The virus is certainly capable of it, but whether the virus could do this more efficiently is an open question. Viruses can almost always get better at virusing, so I expect they could. Antiviral resistance would also make this virus a far more effective pandemic virus, since there is only one other influenza drug that can be used as an alternative. So far, resistance mutations are not common, but that will rapidly change if more people get flu and are treated with NA inhibitors like oseltamivir.
Although NA inhibitors are not the world’s most potent antivirals, they do work to reduce symptom severity and transmission risk if prescribed early in infection (within the first few days). Unfortunately, if they are prescribed too late, they are basically useless and also select for resistance mutants. Large-scale oseltamivir use would undoubtedly result in resistant viruses emerging, which would render this entire class of drugs useless.
Pandemic trait to acquire: virus can spread from cell to cell, NA inhibitor resistance
Virus barrier to overcome: attachment to host cell, NA inhibitors
Risk mitigation: monitor resistance mutations, responsible antiviral drug stewardship
Transmission
A virus won’t become a pandemic if it can’t spread from person to person. In order to do that, it has to be shed in sufficient quantity to infect someone else. A virus’ potential for spreading between hosts is called transmissibility. For flu in humans, it has to not only replicate in the human upper respiratory tract, but infectious virus has to be shed in mucus, saliva, and respiratory aerosols. If it doesn’t do this, the human host is effectively a dead end for the virus.
In order for a virus to become more transmissible, it not only has to meet the conditions above (exposure to naive susceptible hosts, use the human receptor, replicate efficiently, block host antiviral defenses, make new virus particles, and get out of the cell), but it also has to do all these things from the right type of tissue. Flu can be transmitted by a number of different routes. This may determine its potential to spread and may partly explain why the human infections in the US haven’t resulted in even limited transmission between people. Most infected dairy workers report their main symptom is conjunctivitis (pink eye). This doesn’t produce respiratory aerosols since humans don’t breathe through their eyes. That may be one reason why the virus has not yet adapted to human respiratory transmission: it hasn’t had enough opportunity to adapt to tissues of the nose and throat that are involved in breathing.
This could change, especially as H5N1 is certainly capable of infecting the human respiratory tract and flu season is just getting started. Human seasonal flu strains are very well-adapted for human transmissibility, and reassortment with H5N1 could create a pandemic virus with a single co-infection. Transmissibility is dependent on so many variables that it is extremely difficult to predict, but when it happens, the results can be catastrophic. Three out of the last four flu pandemics were caused by recent reassortants, and the fourth (1918) probably was but we don’t have enough sequence data from a century ago to say for sure. The dairy cow outbreak was caused by a recent reassortment between two avian H5N1 viruses. The more infected people there are, the more opportunities there are for reassortment to occur in the tissue type that is most likely to facilitate human to human transmission: the upper respiratory tract.
Although we don’t know how the virus specifically needs to change to acquire efficient transmissibility and thus pandemic potential, we know how to prevent it from doing so: stop humans from becoming infected, especially during flu season. Prevent the virus from adapting to the upper respiratory tract and prevent reassortment with seasonal H1N1 or H3N2, and you prevent an H5N1 pandemic.
Pandemic trait to acquire: virus can spread from person to person
Virus barrier to overcome: “dead end host”
Risk mitigation: prevent and rapidly detect human infections
The path to a pandemic
Cross-species adaptation is really complicated and never is as simple as “we’re one mutation away.” Viruses change and adapt to their hosts, but they don’t become epidemics or pandemics unless the circumstances are right. A good example of this is the dairy cow outbreak in the US.

The dairy cow outbreak caused by a novel reassortant genotype B3.13 H5N1 virus that clearly has the ability to replicate efficiently and shed from bovine mammary tissue, but it never would have spread so widely if circumstances didn’t facilitate it. A single introduction of this virus into cows caused the huge outbreak, which has now infected more than a thousand herds, spilled back to other species, and infected a bunch of human dairy workers. That spread likely occurred through conditions specific to the dairy industry: lots of cows on farms, biosecurity and surveillance practices that didn’t account for influenza virus because we didn’t think bird flu was very good at infecting cows, and frequent interstate transport of lactating cows. We weren’t expecting it, so we didn’t know the risks that needed to be mitigated. We didn’t mitigate them.
We still don’t know specifically which risks to mitigate to keep H5N1 from becoming a pandemic, but this virus is everywhere. We know more generally what we can do to prevent this virus from making the jump and we should do that now. An H5N1 pandemic would be enormously destructive. Not only does it have a higher mortality rate than COVID-19 did at its worst, flu kills children as well as older people, kills or stops production in numerous agriculturally important species of poultry and livestock, and infects tons of wild animals. A H5N1 pandemic would be catastrophic for public health, but also for agriculture, the economy, food security, and the environment. Many more people would die from an H5N1 pandemic than died from COVID-19. I cannot overstate how much of a threat it is to global health and security.
Unfortunately, in the US, the current leadership is taking an aggressively ignorant approach. The elimination of essential functions at CDC further exacerbate this, as do cuts to NIH funding and pandemic preparedness, attacks on science, and what seems to be the Trump administration’s decidedly pro-pathogen stance. These all create circumstances that are conducive to H5N1 “going pandemic,” so we need to end these circumstances. Scientists are often reluctant to get political, and many (wrongly) insist that science should be entirely removed from politics. In this case, if you avoid the politics, you could get a pandemic for your trouble. To stop this virus from adapting to humans, reassorting, and leveling up to cause a pandemic that will make COVID-19 seem like a fond memory. We need to stop enabling the conditions that will allow H5N1 to become a pandemic. As always, we must demand that Congress step in and strongly push for pandemic prevention activities as outlined above.



excellent summary
Angela Rasmussen this is brilliant! I wish you were in charge of this file