Hot Dead Raccoon Summer
More reasons to believe that the lull in bird flu isn't because H5N1 went away. Viruses don't disappear when we stop monitoring them.
Raccoons and other wildlife, like all other beings in this mortal realm, will eventually die for many reasons, including old age, cancer, getting pancaked by a passing vehicle, and infectious disease. This latter cause of death is the one that concerns me.
The most concerning zoonotic virus (transmitted from animals to humans) until 2022 in North American wildlife was rabies virus. Most people don’t think much about rabies because animal health laws in Canada, the US, and Mexico require rabies vaccinations for dogs, since as companion animals with sharp teeth, they present the most significant risk to humans for rabies transmission. Vaccinating pet dogs effectively controls most human cases of rabies, because if less rabies is circulating overall, there are fewer opportunities for cross-species transmission.
But after 2022, another emerging zoonotic virus began to appear with disturbing regularity in North and South American mammals: H5N1 avian influenza virus. The number of mammalian detections has been steadily increasing, considering that a big obstacle to bird flu causing a pandemic is its relatively poor ability to replicate in mammals compared to avian hosts. If the virus doesn’t replicate much, the host will not shed much. If the host doesn’t shed much, the virus won’t be efficiently transmitted (and H5N1 is already inefficiently transmitted between some mammals, including humans, for other biological reasons I will discuss in a moment). If the virus is inefficiently transmitted, it is not capable of causing epidemics or pandemics.
While this may seem reassuring for the time being, influenza viruses can change rapidly. They can gain the ability to infect new cells, tissues, or hosts through mutation (“genetic drift”) and reassortment (“genetic shift”). These genetic changes occur every time that the virus replicates and copies its genome and give the virus the ability to interact more effectively with its host. Viruses are obligate parasites that cannot replicate without a host, so in new hosts, they must adapt or die. The more often a virus replicates in a host, the more the virus will adapt to it according to the evolutionary principle of natural selection. Bird flu, as the name implies, is adapted to replicate and transmit efficiently between avian hosts. Every time H5N1 infects a mammal, it buys a genetic lottery ticket to grow and spread in mammalian hosts. And H5N1 has been buying a lot of these mammalian lottery tickets.
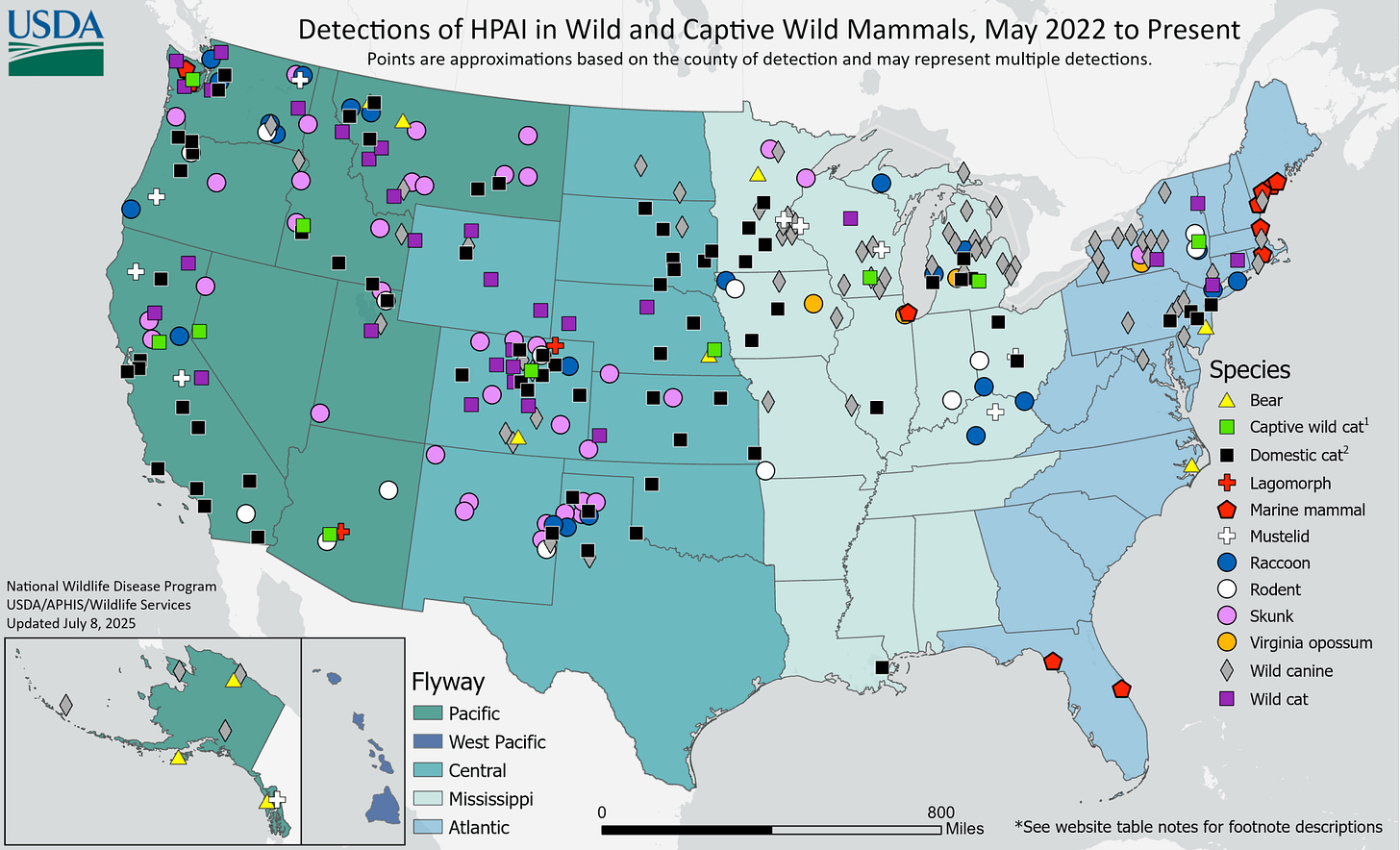
Since H5N1 reached our shores a few years ago, we’ve been finding dead wild mammals that test positive for bird flu. A lot of dead mammals representing many different species. Across the US, there have been bears, cats, exotic cats like lions and tigers in zoos, cougars, bobcats, lynxes, mice, rats, squirrels, rabbits, weasels, minks, skunks, foxes, opossums, coyotes, and raccoons. Up and down both coasts, seals, sea lions, and dolphin corpses have washed ashore. Since most of the wild animals are found incidentally, we are likely missing many more infections that we simply don’t know about.
In many cases, these animals were infected by eating or interacting with infected birds rather than another mammal. Many of these infected mammals are probably evolutionary dead ends and don’t result in the virus being transmitted onward to another mammal. However, analysis of viral genome sequences in outbreaks or die-offs in seals and sea lions, farmed mink, and dairy cows indicates that mammals are transmitting it to other mammals.
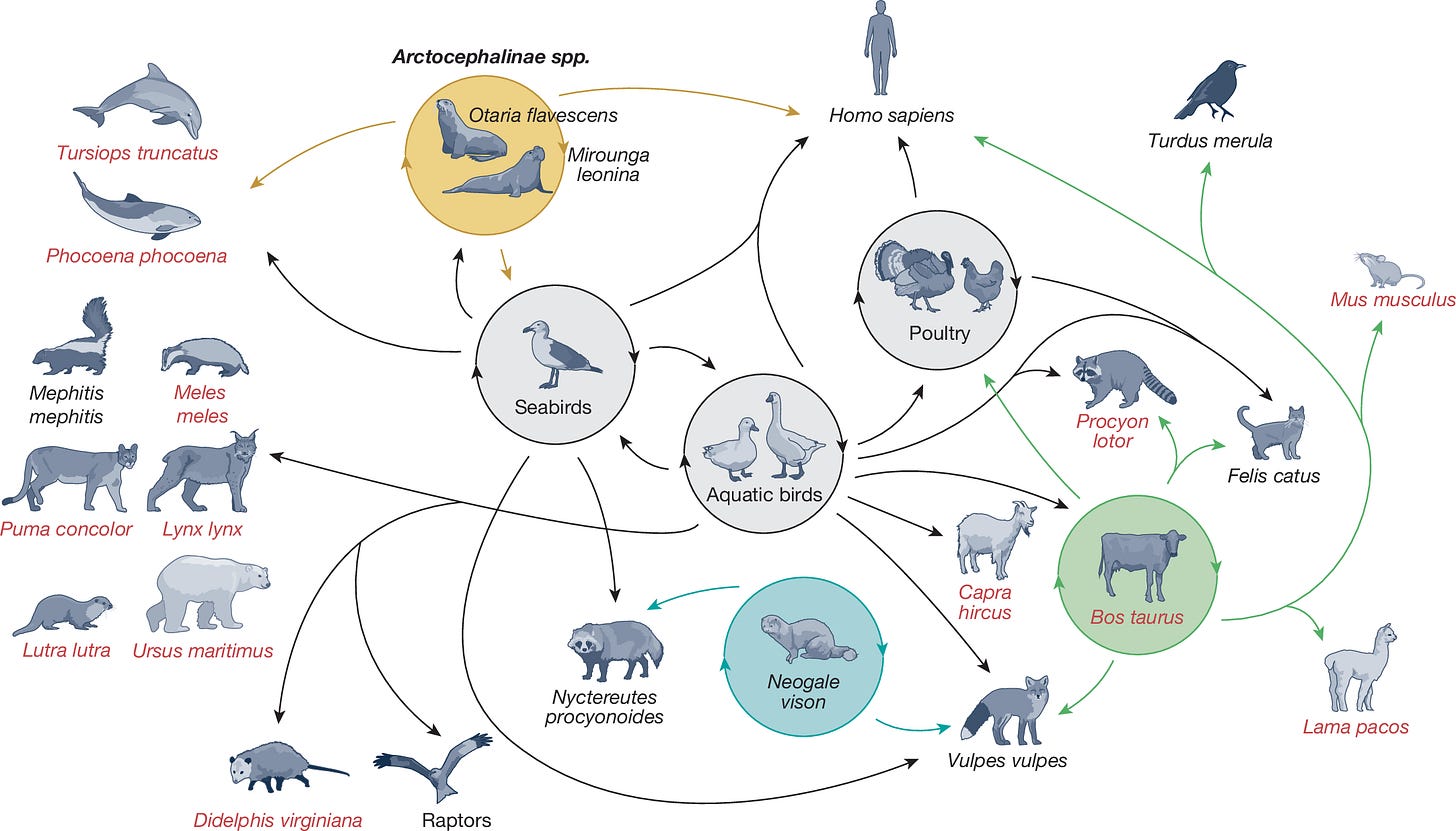
Sustained mammal-to-mammal transmission is not good news, especially when the virus jumps around across a menagerie of mammalian species. The more opportunities that H5N1 gets to adapt to mammalian hosts, the more likely that humans will be among the species of mammals that can be infected.
Why hasn’t H5N1 hit the mammalian jackpot yet?
Any time a virus adapts to a new host, it has multiple obstacles that it must overcome and avian flu is no exception. First, an uninfected host has to encounter a virus in a way that will put them at risk for infection (virologists call this exposure). Then, a virus must get into the host cell in order to infect them (virologists call this susceptibility). Then, the virus must be able to hijack the host cellular machinery to replicate (virologists call this permissivity) and make new virus particles (virologists call this assembly). Often, the virus needs to evade the host cell’s antiviral defenses (virologists call this antagonism). The newly made virions (virus particles) have to find their way back out of the cell (virologists call this egress). Sometimes viruses exit the cell via a route that allows the virus to exit the host and access the external environment (virologists call this shedding). Finally, the virus has to be shed in sufficient quantity via a route that will give it access to a new host (virologists call this transmissibility). All of these processes influence a virus’s inherent capability to make its host sick (virologists call this virulence) or how the interactions between the virus and host cause disease (virologists call this pathogenicity).
There are many influenza A viruses and subtypes (HxNx) and we’ve seen them infect many different species of animals. But this doesn’t mean that H5N1 will be equally competent to overcome all these barriers in all species. Different hosts are susceptible to H5N1 by different infection routes and develop different disease presentations across a broad range of severity. With clade 2.3.4.4.b H5N1 (the type of H5N1 circulating in North America), the same virus can produce very different results in different animal hosts, which in turn get infected by different exposure routes. Cats, for example, develop lethal neurological disease after they consume the virus in raw milk or pet food. Cows can get infected by the respiratory route, but they typically have transient virus shedding without much clinical disease. It appears that much of the dairy cattle transmission is driven by shared milking equipment, since lactating cows shed very high titers of virus in milk. When infected by this intramammary route, they often stop producing milk and can show a range of disease symptoms. Marine mammals like seals may get infected by consuming infected seabirds, but they spread it to each other by unknown routes and suffer mass die-offs from it, showing a range of pathology that suggests both respiratory and neurological disease. Minks and ferrets can be infected by direct contact, inhalation, and consumption. Humans can be infected via the eyes by exposure to raw milk and also by direct contact and inhalation. So far cases of conjunctivitis in dairy or poultry workers have not resulted in severe respiratory disease, possibly because there are not susceptible or permissive cells in the tissues connecting the eye to the lower respiratory tract. However, people exposed by inhalation or contact with infected birds develop severe, often lethal viral pneumonia.
This gets even more complex when comparing different species infected by the same route. One reason it’s thought that human respiratory cases are often severe is receptor distribution. The receptor for influenza is a sugar molecule called sialic acid that comes in multiple forms. Avian viruses like H5N1 use a “bird” version of sialic acid to enter and infect a cell, while human seasonal subtypes like H1N1 or H3N2 use a “human” version. Most species express both kinds of receptors, but they are distributed differently throughout the body. In humans, the “bird” receptor is expressed on the cornea of the eye and deep in the lungs, while the “human” receptor is in the nose, mouth, and upper respiratory tract. This is thought to be why when humans inhale a large dose of H5N1, they get very sick with lower respiratory tract disease, but don’t pass it to other humans since they don’t shed much virus with exhalation. Ferrets have a similar receptor distribution and respiratory physiology, and they also develop comparably severe disease, which is why they are used as a model for human disease. But receptor distribution is not the only determinant of disease severity. Pigs have both bird and human receptors throughout their entire respiratory tract from stem to stern, but they don’t get very sick even though they can grow and shed a lot of virus. And in people and other mammals, there is evidence of mutations associated with adaptation to mammalian hosts besides receptor usage: those that help the virus replicate more efficiently and make the host more permissive, that increase shedding, or increase transmissibility by different routes.
We mostly understand this complexity by studying livestock, companion animals, experimental models, and observational data (such as outbreaks in zoos or animal sanctuaries, fur farms, and in found carcasses). But wild animals are much harder to study and there’s very little funding for broad surveillance in wildlife. Mammals that are infected but don’t get very sick are mostly invisible to us, and the dead animals we do find likely represent a small sample of the total amount that are infected. We don’t know about exposure and transmission routes for many of them. We don’t know how the unique selection pressures of each host species will drive host-specific adaptation, or how convoluted cross-species transmission networks will influence virus evolution. We do know that the virus will continue to evolve. We also know that H5N1 can infect many different species. Given enough opportunity and experience with these other mammals, it might get better at infecting humans. Growth in one species could lead to viruses that are capable of infecting via different exposure routes, using human receptors for entry more efficiently, grow and shed at higher levels, cause more disease, or are more capable of transmission.
What we don’t know might kill us
There are vast gaps in our understanding of how H5N1 will adapt as it passes through all these mammals. We don’t know how many wild mammals are infected or how many get sick or what populations of animals these viruses are circulating in. What we do know is that we’re finding more dead mammals across America that test positive for H5N1.
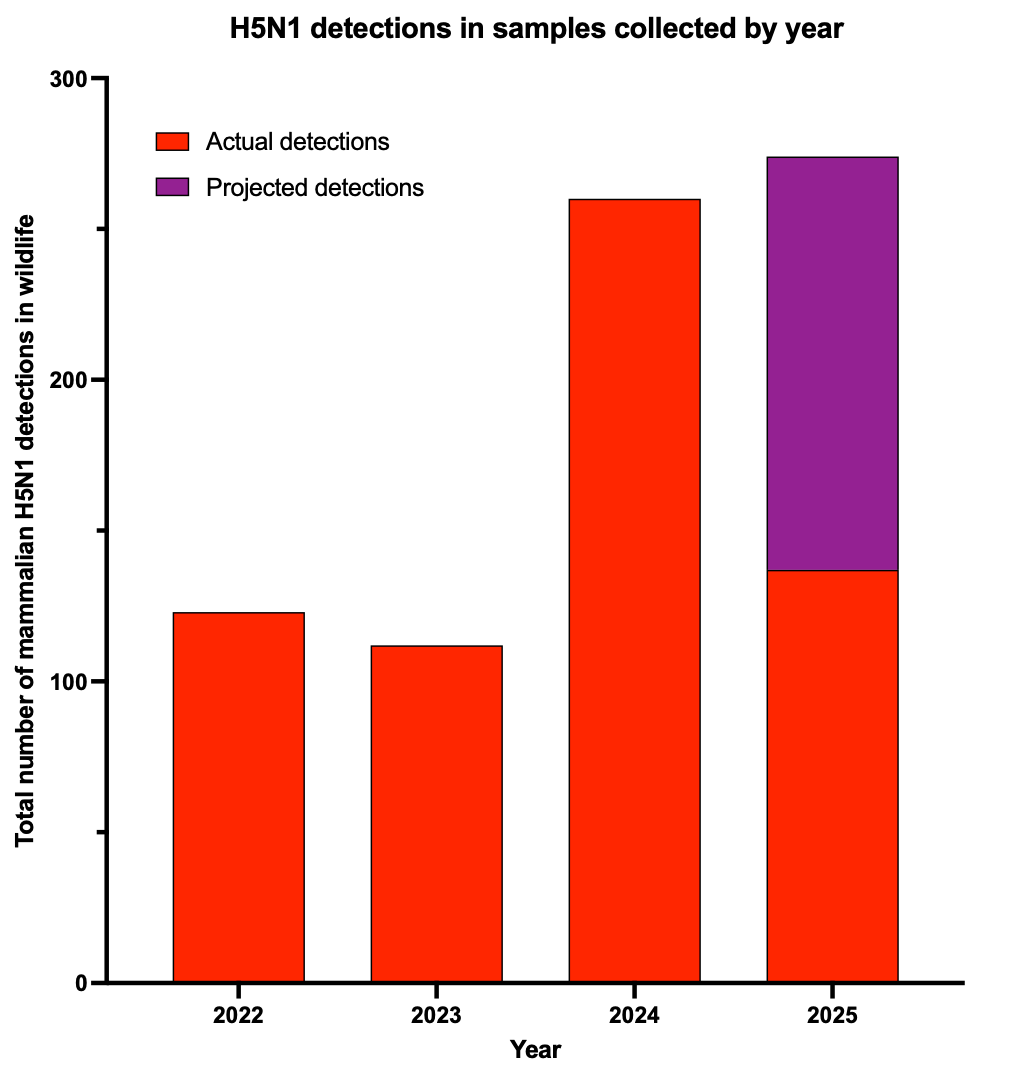
In 2024, we detected an increase in the number of H5N1-infected wild mammals. If dead mammals continue to turn up, we are on track to exceed that this year (if testing and surveillance continues).
One thing is clear, however. H5N1 has not gone away. We might not have detected any human cases in the past few months (in part because we aren’t really looking for it anymore), but that doesn’t mean it isn’t infecting mammals any more. It clearly is killing some wild animals and likely infecting many more that we simply aren’t seeing. That is the part that scares me, as I discussed back in March on 60 Minutes.
We know from the mammals tested so far, as well as from human sequences, that mammalian hosts select for mammal-adapted viruses. We don’t know where these animals are, how many are infected, how they are getting infected, and what or who these viruses are going on to infect. While I hypothesize that a true pandemic-capable H5N1 would likely emerge from a human or a pig, I can’t exclude that this could also occur in other mammals. Another scenario is that a virus adapted to other mammals could be just enough to infect humans that it could establish transmission chains and further adapt to a human host with each new infection. As the virus adapts to human hosts, it would likely become more adapted to using the human receptor, replicate to higher levels, assemble and shed more virus, and more readily transmit between human hosts. It also may become better at evading human antiviral defenses and become more or less pathogenic.
Although we don’t know about seasonality, ecology, or transmission patterns of H5N1 spread in various wild mammals, we do know that in several months, human flu season will begin. As a result, H1N1 and H3N2 viruses that are well-adapted to humans will increase in circulation. When this occurs, there will be new opportunities for reassortment (mixing up segments from different viruses to make new ones with unknown properties). Reassortment of avian viruses with human or swine viruses can allow rapid host adaptation. This occurred for the 2009 H1N1 pandemic and likely for the 1918 H1N1 pandemic, as well (there are not enough 1918 genomic sequencing. Some of these wild mammals are susceptible to other subtypes of influenza virus, including seasonal human viruses. If reassortment occurs in wildlife, potential pandemic viruses could begin to circulate without us knowing about it. And if this virus begins to circulate widely across many mammals, it could emerge into the human population without warning.
Nobody, including me, can say for sure if or when or under what circumstances H5N1 will acquire these abilities and cause a pandemic. There are so many confounding variables that we can’t quantify or adjust for. How many mammals are infected? Where are they? How do they interact with each other and other animals? Do they shed much virus? Are they capable of transmission? Do they get sick? Do they clear the infection or can it be persistent? What is the risk of reassortment? We can only try to estimate relative risk based on the very incomplete data that we have.
But one thing is certain: H5N1 continues to circulate widely in North American mammals and the risk remains high. Bird flu could still emerge at any time, but that risk will increase further when human flu season returns to the northern hemisphere. If we aren’t paying attention, the next mammalian species at risk will be our own.





And of course the CDC is all over this under RKJr and his anti vax cabal.
Let’s hope this waits until a reality based leadership has began rebuilding the CDC.
Thanks very much for sharing this. You have a talent for explaining these concepts clearly to neophytes. And, yes, I did behold the graph and was amazed! 😉